Paranoia [ˌpærəˈnɔɪ.ə] (adjective: paranoid [ˈpærə.nɔɪd]) is a thought process believed to be heavily influenced by anxiety or fear, often to the point of irrationality and delusion. Paranoid thinking typically includes persecutory beliefs, or beliefs of conspiracy concerning a perceived threat towards oneself. (e.g. "Everyone is out to get me.") Making false accusations and the general distrust of others also frequently accompany paranoia. For example, an incident most people would view as an accident or coincidence, a paranoid person might believe was intentional. However, just because an individual is paranoid does not necessarily mean his or her suspicions are false, as noted in Catch-22: "Just because you're paranoid doesn't mean they aren't after you."
Historically, this characterization was used to describe any delusional state. In modern colloquial use, the term "paranoia" is sometimes misused to describe a phobia. The general lack of blame in phobia disorders sharply differentiates the two. In other words, fearing that something bad or harmful might happen does not in itself imply paranoia. Rather, with paranoia there is an irrational fear of malice by others (excepting rare cases of schizophrenia).
The word paranoia comes from the Greek "παράνοια" (paranoia), "madness"[1] and that from "παρά" (para), "beside, by"[2] + "νόος" (noos), "mind".[3] The term was used to describe a mental illness in which a delusional belief is the sole or most prominent feature. In an original attempt at classifying different forms of mental illness, Kraepelin used the term pure paranoia to describe a condition where a delusion was present, but without any apparent deterioration in intellectual abilities and without any of the other features of dementia praecox, the condition later renamed "schizophrenia". Notably, in his definition, the belief does not have to be persecutory to be classified as paranoid, so any number of delusional beliefs can be classified as paranoia. For example, a person who has the sole delusional belief that he is an important religious figure would be classified by Kraepelin as having 'pure paranoia'. According to Phelan, M. Padraig, W. Stern, J (2000)[4] paranoia and paraphrenia are debated entities that were detached from dementia praecox by Kraepelin, who explained paranoia as a continuous systematized delusion arising much later in life with no presence of either hallucinations or a deteriorating course, paraphrenia as an identical syndrome to paranoia but with hallucinations. Even at the present time, a delusion need not be suspicious or fearful to be classified as paranoid. A person might be diagnosed as a paranoid schizophrenic without delusions of persecution, simply because their delusions refer mainly to themselves.
Everything Dr. Russel Blaylock is 100% documented. The drinking water is poisoned/a mass medication, with sodium fluoride. Aspartame a neuro-toxin is added to various food products.
The artificial sweetener aspartame has been the subject of several controversies since its initial approval by the U.S. Food and Drug Administration (FDA) in 1974. The FDA approval of aspartame was highly contested,[1] with critics alleging that the quality of the initial research supporting its safety was inadequate and flawed and that conflicts of interest marred the approval of aspartame.[2][3][4] In 1987, the U.S. Government Accountability Office concluded that the food additive approval process had been followed properly for aspartame.[2][5] In spite of this, critics such as activist Betty Martini[6] have promoted undocumented claims that numerous health risks (such as multiple sclerosis, systemic lupus, methanol toxicity, blindness, spasms, shooting pains, seizures, headaches, depression, anxiety, memory loss, birth defects and death[7]) are associated with the consumption of aspartame in normal doses. These health risk claims have been examined and debunked by numerous scientific research projects, and are also generally dismissed by governments and major health and food safety organizations.[2][8][9]
Publicity of this controversy has been spread through an elaborate health scare[10] and "Internet smear campaign"[11] involving hoax[10][12][11] e-mails repeating Betty Martini's widely circulated conspiracy theory. Her undocumented claims are still repeated by thousands of self-published Web sites.
Aspartame has been found to be safe for human consumption by more than ninety countries worldwide,[13][14] with FDA officials describing aspartame as "one of the most thoroughly tested and studied food additives the agency has ever approved" and its safety as "clear cut".[4] The weight of existing scientific evidence indicates that aspartame is safe as a non-nutritive sweetener.
In 1997, due to public concerns the UK government introduced a new regulation obliging food makers who use sweeteners to state clearly next to the name of their product the phrase "with sweeteners".[33]
In 2007, the Indonesian government considered banning aspartame.[34] In the Philippines, the small political party Alliance for Rural Concerns introduced House Bill 4747 in 2008 with the aim of having aspartame banned from the food supply.[35] The U.S. state of New Mexico introduced a bill to ban aspartame in 2007,[36][37][38] and Hawaiian legislators signed a 2009 resolution asking the FDA to rescind approval.[39] In March 2009, the California OEHHA identified aspartame as a chemical for consultation by its Carcinogen Identification Committee, in accordance with California state Proposition 65.[40]
In 2007, the UK supermarket chains Sainsbury's,[41] M&S,[42] and Wal-Mart subsidiary Asda,[43] announced that they would no longer use aspartame in their own label products.[44] In April 2009, Ajinomoto Sweeteners Europe, the makers of Aspartame in Europe, responded to Asda's "no nasties" campaign by filing a complaint of malicious falsehood against Asda in the English courts.[45][46] In July 2009, Asda initially won the legal case after the trial judge construed the "no nasties" labelling to "not mean that aspartame was potentially harmful or unhealthy".[47][48] The decision was reversed in June 2010, upon appeal,[49] and was settled in 2011 with ASDA removing references to aspartame from its packaging.[50]
In 2009, the South African retailer Woolworths announced it was removing aspartame from its own-brand foods.[51]
In 2010, the British Food Standards Agency launched an investigation into aspartame amid claims that some people experience side-effects after consuming the substance. A significant proportion of volunteers participating in the study are those who have claimed to experience side-effects.[52]
In September 2011, the European Food Safety Authority (EFSA), which is due to release the findings of its full re-evaluation of aspartame in September 2012, made all 600 datasets it is using in its full re-evaluation available publicly. This includes previously unpublished scientific data, "including the 112 original studies on aspartame which were submitted to support the request for authorisation of aspartame in Europe in the early 1980s.
By 1984, three years after its initial approval for use in tabletop sweeteners and dry food, U.S. consumption of aspartame had already reached 6.9 million pounds per year. This number doubled the following year, and continued to climb well into the 90's.
According to statistics published by Forbes Magazine [i] based on Tate & Lyle estimates, aspartame had conquered 55 percent of the artificial sweetener market in 2003. One of the driving factors behind aspartame's market success is the fact that since it is now off patent protection, it's far less expensive than other artificial sweeteners like sucralose (Splenda).
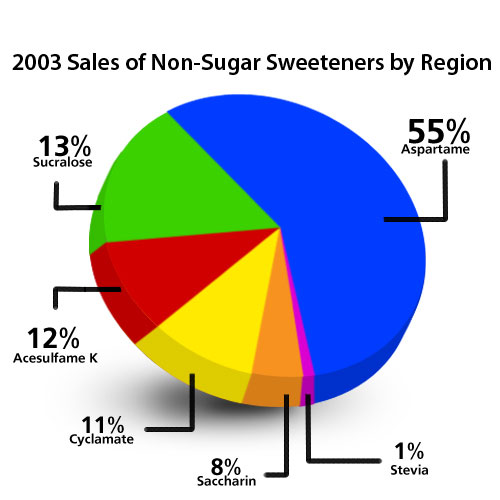
Today, the statistics on the aspartame market are being kept so close to the vest, it has proven to be virtually impossible to find current data on usage, unless you're willing to pay tens of thousands of dollars for a market analysis reports and I felt there were better uses for the money than to purchase the answer to that question.
However, a 2009 FoodNavigator article[ii]cites the current global market for aspartame as being less than 37.5 million pounds and worth $637 million.
According to aspartame.org [iii], diet soda accounts for 70 percent of the aspartame consumed. A 12 ounce can of diet soda contains 180 mg of aspartame, and aspartame users ingest an average of 200 mg per day.
However, it can be quite difficult to calculate just how much you're really ingesting, especially if you consume several types of aspartame-containing foods and beverages. Dosing can vary wildly from product to product. For example, the amount of aspartame will vary from brand to brand, and from flavour to flavour. Some can contain close to twice the amount of aspartame as others, and some contain a combination of aspartame and other artificial sweeteners.
Interestingly, aspartame consumption now seems to have stalled, and there is some indication it may even be on the decline. Perhaps sufficient numbers of people are finally waking up to the unsavory truth about this chemical sweetener. It is my intention to educate you about the truth of this harmful and toxic ingredient and drive sales down even further. I have no ulterior motives other than to warn you so that you can protect your and your family's health, and I sell no competing products.
The only alternative sweetener I recommend is natural stevia, especially the flavoured ones which avoid many of the aftertaste objections some people have about using stevia. It is interesting to note that the powerful food industry has made it illegal to sell natural stevia as a sweetener. If I recommended to use stevia as a sweetener and sold it, the government would immediately file criminal charges and confiscate our product.
On December 17, 2008, the FDA did grant GRAS (Generally Recognized as Safe) status to rebaudioside , which is one component of the whole stevia plant, and this specific purified component of stevia may be used as a food additive and sold as an alternative sweetener. Examples of include Truvia and Purevia. The jury is still out, however, on whether consuming this one component of stevia is as safe as consuming extract from the whole plant, as all the synergistic, protective factors have been removed in these refined products.
Thankfully there is a loophole that allows vendors to sell extract of whole stevia as a dietary supplement. Since virtually everyone knows it is a sweetener it doesn't have to say it on the label, so you can still bypass this industry initiated censorship.
Ajinomoto, one of the leading aspartame manufacturers in the world next to NutraSweet, actually rebranded aspartame to AminoSweet [iv] last year, in order to dissociate itself from the negative associations of aspartame.
It also wanted to "remind the industry that aspartame tastes just like sugar, and that it's made from amino acids -- the building blocks of protein that are abundant in our diet," -- as opposed to a concoction of chemicals never before consumed by man, some ingredients of which are more toxic than others. They will probably deceive some consumers with this newer, more sweetly innocent name that does not bear the same controversial past as the word "aspartame." But I sincerely doubt they'll fool anyone even remotely aware of its dangers.
Aspartame can already be found in some 6,000 food products and beverages, and the list is about to get even longer, I'm sure, as Ajinomoto announced a global R&D alliance agreement with Kellogg Company [v] earlier this month.
Researchers Continue to Contest 'the Most Contested' FDA Approval in History
Concerned scientists and researchers fought and were successful in keeping aspartame out of the food supply for over ten years, ever since it was first considered as a potential food additive, and many of those still alive continue to speak out against it today.
If we fail to learn from history we are doomed to repeat the mistake we made. Many readers have long forgotten what the 60-Minutes' correspondent Mike Wallace stated in his 1996 report on aspartame - available to view in this 2009 article - that the approval of aspartame was "the most contested in FDA history." And for good reason.
At the time, independent studies had found it caused brain cancer in lab animals, and the studies submitted by G.D. Searle to the FDA for the approval were quickly suspected of being sloppy at best...
In that 60-Minutes video, former Senator Howard Metzenbaum states:
"According to the FDA themselves, Searle, when making their presentation to the FDA, had willfully misrepresented the facts, and withheld some of the facts that they knew would possibly jeopardize the approval."Metzenbaum's staff investigated the aspartame approval process. He goes on to explain that:
"FDA officials were so upset they sent the file to the U.S. Attorney's office in Chicago for the purposes of presenting it to the grand jury as to whether or not there should be indictments. But it wasn't presented. It was delayed."Samuel Skinner, the U.S. attorney who led the grand jury probe ended up withdrawing from the case when he entered into job discussions with Searle's Chicago law firm, Sidley & Austin - a job he later accepted. Subsequently, the investigation stalled until the statute of limitation ran out, at which point the investigation against Searle was dropped.
For more details on the story of how aspartame made it through the FDA approval process despite warning signs of potential health hazards and alleged scientific fraud, please watch the 60-Minutes report, as Wallace does a nice job of summarizing an otherwise long story.
There are a number of well-written books on the market that detail the twists and turns of this part of history. This Harvard law summary of the legal wrangling [vi] that took place is also a worthwhile read.
Those who claim that aspartame watch-dogs are somehow engaged in conspiracy theories, perhaps do not understand the word "conspiracy," the simplest definition of which is: "a secret agreement between two or more people to perform an unlawful act." In the case of aspartame, it sure does look as though it was a conspiracy -- by G.D. Searle & Co., to get a tremendously profitable product to market, no matter what the potential cost in terms of human health.
The FDA itself suspected Searle had unlawfully produced "evidence" to support its claims of safety, and FDA officials were sufficiently disturbed by what they received to launch its first-ever criminal investigation. A section in the Harvard Law School summary on the history of aspartame states:
"Another study that engendered severe criticism from the Department of Health Education and Welfare was the 46- week toxicity study performed on the hamster.How's that for assurance?
Although the data appears to be faulty and incomplete, Searle argues that any falsehood in the study is not material to the appraisal of the safety of aspartame."
"...In addition to criticizing the study as a whole, the Department alleges that Searle violated Title 18, Section 1001 by falsifying data. The report alleges that the testing ran into problems and instead of correcting them, Searle covered the problem up."FDA toxicologist, M. Jacqueline Verrett, Ph.D., discussed what she knew about some of these concerns in her testimony before Congress on November 3, 1987 (S.hrg;100-567).
Verrett's individual testimony is reprinted here, in which she states:
"From 1957-1977 I was employed as a Biochemist/Toxicologist in what is now designated the Center for Food Safety and Applied Nutrition of the Food and Drug Administration. ... In the early l970's, I examined the animal studies submitted by G. D. Searle and Co. on aspartame prior to the initial approval by FDA in l974... these studies raised numerous questions in a number of areas that needed to be resolved before approval of aspartame for any food additive use.Verrett goes on to point out a number of the "deficiencies and improper procedures encountered" by her investigative team, which included but were not limited to:
In 1977 I served as a member of an FDA team... charged with examining three studies... to determine if they were 'authentic'. I wish to emphasize at this point that we were specifically instructed not to be concerned with, or comment upon, the overall validity of the study, this was to be done in a subsequent review carried out at the Bureau level.
It is apparent that that review, on a point by point basis, discarded or ignored the problems and deficiencies outlined in this Team report, and concluded that, even in toto, these problems were insufficient to render the study invalid. It also appears that the serious departures from acceptable toxicological protocols that were noted in the reevaluation of these studies were also discounted."
*Animals were not permanently tagged to avoid mix-ups
*Tumors were removed and the animals returned to the study
*Animals were recorded as dead, but subsequent records, after varying periods of time, indicated the same animal was still alive (almost certain evidence of mix-ups)
*Many animal tissues were decomposed before any postmortem examinations were performed
"Almost any single one of these aberrations would suffice to negate a study designed to assess the safety of a food additive," Verrett said, "and most certainly a combination of many such improper practices would, since the results are bound to be compromised. It is unthinkable that any reputable toxicologist, giving a completely objective evaluation of data resulting from such a study, could conclude anything other than that the study was uninterpretable and worthless,and should be repeated.
This is especially important for an additive such as aspartame, which is equally vital since DKP is a major breakdown product of aspartame in liquid media.
Not only is aspartame being used in the absence of basic toxicity information, but there is also no data to assess the toxicity of the interactions of DKP with the excess phenylalanine generated, with any other metabolite of aspartame, and its interactions with other additives, drugs, or other chemicals which may be present simultaneously in persons exposed to high levels of DKP in presweetened liquids such as diet drinks."
Many critics are using the lack of toxicity data as proof that aspartame is safe, when in fact aspartame appears to have been approved WITHOUT such data - which in my opinion is just another sign of aspartame's inherent LACK of empirical safety record...
Which brings us to a crucial point.
If you do not know this fact, you may never be able to extract the truth, because the 200+ studies that form the basis of aspartame's multiple FDA approvals DO exist. Those studies were published, and are quite easy to find as they're cited by every single conventional health agency and every single aspartame peddler across the world. No one is trying to refute the fact that they exist.
However, they were ALL funded by the aspartame industry.
And guess what happens when you remove corporate interest and influence from the equation...
All Industry-Funded Studies Give Aspartame Clean Bill of Health, While Majority of Independent Research Find Indications of Hazards
A 1996 review of 165 studies [vii] [viii] believed to be relevant to human safety, by Dr. Ralph G. Walton, a professor of Clinical Psychiatry, showed a remarkable discrepancy between study results and their source of funding.
Of the 165 studies, 74 had industry related funding (such as Searle, Nutrasweet®, Ajinomoto, and the International Life Sciences Institute Nutrition Foundation), and 91 were independently funded.
Of those:
*100 percent of the industry funded studies supported aspartame's safety, while
*92 percent of the independently funded studies identified at least one potential health concern
However, Dr. Walton also pointed out that of the seven remaining non-industry funded studies, which supported aspartame's safety, six were done by the FDA, and the seventh was a literature review of mostly industry sponsored research.
Considering the long-standing revolving door between various industries (especially Monsanto, which acquired G.D. Searle in 1985) and the FDA, it's questionable as to whether an FDA study can be considered truly "independent," even though they were counted as independent in Walton's review.
If you give that concern any merit, you'd more or less be looking at 100% of industry related studies claiming aspartame to be safe, and 100% of independent studies flagging some sort of health concern.
If this doesn't make you raise an eyebrow, then no need to read any further. You've slipped comfortably into the all-accepting fold of corporate self-interests, created by massively successful propaganda and public relations efforts, backed by powerful political lobbying.
Only you can decide whether or not you find this discrepancy to be acceptable evidence of rigorous scientific inquiry.
If it makes you question the validity of aspartame's "100% safe" designation, then read on...
Your Brain on Aspartame
In the Sweet Misery video above, Dr. Russell Blaylock, a recently retired board-certified neurosurgeon and author of the book Excitotoxins: The Taste That Kills, says that because aspartame is "a poison that affects protein synthesis; affects how the synapses operate in the brain, and affects DNA, it can affect numerous organs. So you can get many different symptoms that seem unconnected."
However, "when looking at the list of symptoms submitted to the FDA, most of them are neurological," Dr. Blaylock says.
He's referring to a Department of Health and Human Services report that categorizes 10,000 adverse reaction reports logged by the FDA (Department of Health and Human Services Quarterly Report on Adverse Reactions Associated with Aspartame Ingestion, DHHS, Washington, DC, October 1, 1986), published here in a 24-page primer on aspartame by Donald Harkins [ix], the former editor and publisher of the Idaho Observer.
Two years prior to that, a CDC MMWR dated November 2, 1984 [x] , discusses several hundred adverse reaction reports received, and at that time, the majority -- 67 percent - of complainants also reported neurological/behavioral symptoms.
Some of the most commonly reported neurological symptoms include:
*Headaches
*Changes in behavior or mood
*"Fuzzy" thinking
*Seizures
*Depression[xi]
A 1987 study published in the journal Environmental Health Perspectives [xii] states:
"If only 1% of the 100,000,000 Americans thought to consume aspartame ever exceed the sweetener's ADI, and if only 1% of this group happen coincidentally to have an underlying disease that makes their brains vulnerable to the effects of an aspartame-induced rise in brain phenylalanine levels, then the number of people who might manifest adverse brain reactions attributable to aspartame would still be about 10,000, a number on the same order as the number of neutrally related consumer complaints already registered with the FDA and other federal agencies."
[Note: the ADI for aspartame is 50 mg/kg of body weight in the US. ADI in Europe and Canada is 40 mg/kg of body weight. ]Published in the European Journal of Clinical Nutrition in 2008 [xiii], a South African study offers further information on the potential workings of aspartame on your brain:
"Phenylalanine plays an important role in neurotransmitter regulation, whereas aspartic acid is also thought to play a role as an excitatory neurotransmitter in the central nervous system. Glutamate, asparagines and glutamine are formed from their precursor, aspartic acid.There has been loads of conflicting "science" regarding the metabolism of methanol. The emerging evidence suggests that it may be a toxic poison that is one of the leading contributing factors for MS, and that some of the research is subsidized by the producers of methanol to make it appear less harmful.
Methanol, which forms 10 % of the broken down product, is converted in your body to formate, which can either be excreted or can give rise to formaldehyde, diketopiperazine (a carcinogen) and a number of other highly toxic derivatives.
Previously, it has been reported that consumption of aspartame could cause neurological and behavioral disturbances in sensitive individuals. Headaches, insomnia and seizures are also some of the neurological effects that have been encountered, and these may be accredited to changes in regional brain concentrations of catecholamines, which include norepinephrine, epinephrine and dopamine.
The aim of this study was to discuss the direct and indirect cellular effects of aspartame on the brain, and we propose that excessive aspartame ingestion might be involved in the pathogenesis of certain mental disorders (DSM-IV-TR 2000) and also in compromised learning and emotional functioning."
I hope to an interview in the near future with an expert to review and clarify these details.
Aspartame and Headaches
The Sweet Misery documentary also includes Dr. H.J. Roberts M.D., a board-certified internist and author of Aspartame Disease: An Ignored Epidemic, who does a fine job of explaining, in layman's terms, what aspartame is made of, and how patients have, and can, test their vulnerability to this chemical using cessation and rechallenge. (I will also offer further suggestions on how to do this at the end of this article.)
This type of anecdotal evidence, which critics love to dismiss as silly nonsense, can nonetheless be invaluable to the individual in question, as you can clearly discover whether there's a direct cause and effect on your body from consuming aspartame.
Dr. H.J. Roberts is one of several expert investigators on aspartame and has testified before congress on the topic of aspartame safety.
In a document titled, Professional Opinion of H.J. Roberts, M.D., F.A.C.P., F.C.C.P., Concerning Headaches Caused by the Use of Products Containing Aspartame, he states that:
"People who suffer aspartame-induced headache are likely to encounter denial of this condition by physicians, the FDA and manufacturers. This situation is largely influenced by "negative scientific studies" sponsored by corporate interests.One study commonly cited by industry to refute the claim that aspartame causes headaches is the 1987 NEJM study [xiv] which concluded that "aspartame is no more likely to produce headache than placebo."
I have repeatedly challenged the nature of such studies, especially when the aspartame was administered as capsules or freshly-prepared cool solutions rather than "real world" products, namely soft drinks and other products sold in markets that undergo changes on exposure to high temperature or with storage of more than one or two months."
However, this study, again, has financial ties to Monsanto (owner of G.D. Searle), and the aspartame was given in capsule form, for one day...
There are a number of studies that point to concerns related to aspartame's detrimental impact on neurological function.
Under Sources above, you will find a link to a page on my site where I've created a list of studies, sorted by the health concern they pertain to, and headaches is just one of many potential concerns. That page is evolving, and I will continue to add to it as I find more relevant studies.
In my follow-up article, I will foray into a couple of the other health problems associated with aspartame.
Taking the Precautionary Principle into Account
Any good scientist, and any skeptic worth their own weight, would follow the evidence to its logical conclusion, no matter where it leads. Unfortunately, we have overwhelming evidence showing that it's nearly impossible to be impartial when your paycheck is on the line. Any corporation that pays you to investigate their product wants you to produce favorable results, and we know that powerful corporations can make these desires well understood by those who work for them.
Likewise, any good doctor or health professional would adhere to the Hippocratic Oath that says, "First, do no harm." Yet here we have a chemical sweetener being added to some 6,000 food products, which, due to its sheer prevalence and fervent backing by the conventional medical industry and health agencies, has the potential to harm a vulnerable section of the population.
You're told it is perfectly safe. (Unless you have a genetic disease called phenylketonuria (PKU), which prevents you from digesting the amino acid phenylalanine. An estimated 1 out of every 15,000 people are born with PKU. This is why aspartame containing products bear a warning label stating the product contains phenylalanine.)
But do you know whether or not you have phenylketonuria, or are part of any other "generally vulnerable" group of people?
Wouldn't you want to know if you might be at risk?
And if you knew you were vulnerable to its toxic effects, would you still consume high amounts of aspartame?
If you were not, but you knew that a family member or friend was part of that vulnerable subgroup, would you warn them?
These are simple questions that tend to get completely lost in the pro- versus anti-aspartame debate.
Do you think it's acceptable to willfully sacrifice those who are more vulnerable by issuing no warnings whatsoever? And worse -- pulling the wool over their eyes and saying aspartame has no related health hazards whatsoever, even at very high amounts?
I think not.
Consider the 1986 review of 231 adverse reactions to aspartame [xv], which found "no clear symptom complex that suggests a widespread public health hazard associated with aspartame use." Yet in the following sentence, the researchers admit that:
"...in some case reports... the symptoms may be attributable to aspartame in commonly-consumed amounts. The systematic application of pre-defined review criteria, such as those described here, to monitor consumer complaints related to food additives will help identify products that warrant more focused clinical studies."Staunch aspartame promoters pay no attention to that part - the part that states a certain number of individuals may indeed suffer health consequences, even from commonly-consumed amounts.
They also pay no attention to the fact that this review occurred a mere three years after the US became saturated with aspartame-containing beverages. Today we have thousands upon thousands of adverse reaction reports, anecdotal reports, and physician's case histories...
These people are indeed being sacrificed, without remorse whatsoever, by those hiding behind supremely biased, profit-driven, industry-funded research.
The conventional medical establishment and our health agencies are frightfully resistant to the possibility that aspartame may have anything to do with health problems - after all, aspartame is FDA approved and has been "safely used" for years!
I have one word for you - Vioxx.
Just one of a multitude of FDA-approved products that -- lo and behold - killed tens of thousands of people while the establishment reiterated the industry-funded "scientific evidence" that was the basis for its widespread use.
Are Your Health Problems Related to Aspartame Consumption?
You might not realize you're having a reaction to aspartame. In fact, most people don't make the connection, and a tremendous amount of time and money is spent by aspartame "reactors" (people sensitive to the chemical), trying to find out why they are sick.
To determine if you're a reactor, take the following steps:
1. Eliminate all artificial sweeteners from your diet two weeks. (Note: If you typically consume aspartame in caffeinated drinks, you'll want to gradually reduce your intake in order to avoid caffeine withdrawal symptoms.)
2. After two weeks of being artificial sweetener-free, reintroduce aspartame in a significant quantity (about three servings daily) and avoid other artificial sweeteners during this period.
3. Do this for one to three days and notice how you feel, especially as compared to when you were consuming no artificial sweeteners.
4. If you don't notice a difference in how you feel after re-introducing aspartame, it's a safe bet you're able to tolerate aspartame acutely, meaning your body doesn't have an immediate, adverse response. However, this doesn't mean your health won't be damaged in the long run by this chemical and its breakdown products.
I'm not trying to deny anyone the pleasure of life that is generated from consuming sweets. However, to promote aspartame to the population at large, without warning that a certain percentage of people may suffer terribly from its consumption is a reckless, irresponsible ethical breech, and clearly contributes to much unnecessary suffering in exchange for hundreds of millions of dollars of profit..
In the end it's up to you to decide what you want to put into your body. Just make it an educated decision.
Rofecoxib (
Rofecoxib gained widespread acceptance among physicians treating patients with arthritis and other conditions causing chronic or acute pain. Worldwide, over 80 million people were prescribed rofecoxib at some time.[1]
On September 30, 2004, Merck voluntarily withdrew rofecoxib from the market because of concerns about increased risk of heart attack and stroke associated with long-term, high-dosage use. Merck withdrew the drug after disclosures that it withheld information about rofecoxib's risks from doctors and patients for over five years, resulting in between 88,000 and 140,000 cases of serious heart disease.[2] Rofecoxib was one of the most widely used drugs ever to be withdrawn from the market. In the year before withdrawal, Merck had sales revenue of US$2.5 billion from Vioxx.[3]
Rofecoxib was available on prescription as tablets and as an oral suspension. It was available by injection for hospital use.
Is it safe to mix fluoridated tap water with infant formula?
I've heard that too much fluoride can harm a baby's teeth.
Answer
from Jay L. Hoecker, M.D.
It's considered safe to use fluoridated tap water to prepare infant formula.Exposure to fluoride during infancy helps prevent tooth decay. However, regularly mixing powdered or liquid infant formula concentrate with fluoridated water may increase your child's risk of developing faint white markings or streaks on the teeth — a sign of mild enamel fluorosis. Fluorosis is a cosmetic issue that affects both baby teeth and permanent teeth while they're forming under the gums. In children younger than age 8, combined fluoride exposure from all sources — water, food, toothpaste and other products — contributes to fluorosis.
If you're concerned about fluorosis, you can minimize your baby's exposure to fluoride by using ready-to-feed formula. You can also alternate using tap water and nonfluoridated water for formula preparation, or mix powdered or liquid infant formula concentrate with low-fluoride water most or all of the time. However, if you use only nonfluoridated water — such as purified, demineralized, deionized or distilled bottled water — to prepare your baby's formula, your baby's doctor may recommend fluoride supplements beginning at age 6 months.
Q. Can I use warm or hot water from the tap to make my baby's formula? Cassie, Dallas, Texas
A. Since few babies like cold baby formula and you do usually have that extra step of warming the formula after you make it, using warm or hot water to start with from the tap does seem like it could save an extra step. You shouldn't do it though.
Instead, follow the directions on your baby's formula package and if using tap water, start with cold tap water. According to the U.S. Environmental Protection Agency, you should "never cook or mix infant formula using hot water from the tap."
What's wrong with hot tap water? Many homes have plumbing with lead or lead solder and hot water can concentrate the lead. Running the water for 15 to 30 seconds and only using cold water can help reduce your baby's exposure to lead from tap water.
Boiling the water doesn't get rid of the lead either. Many home water filters, including pitcher and faucet filters, do remove lead from drinking water though.
The American Academy of Pediatrics doesn't offer any formal advice on the subject either. The latest book on newborns that they published, Heading Home with Your Newborn: From Birth to Reality, does say that "you may want to use boiled or purified (bottled or filtered) water, at least in the first month or two."
The main problem with that statement is that purified, bottled, or filtered water, even the brand Nursery Purified Water, isn't sterile, so isn't necessarily any safer than tap water that hasn't been boiled first. Bottled and filtered water should have fewer impurities and contaminants, including lead, but could still have harmful bacteria, which was the whole reason you were supposed to boil tap water when making baby formula in the first place.
And there is no research which states that doing anything special to the water that you use for your baby's formula "in the first month or two" is helpful or does anything at all. That advice is likely based on the fact that younger babies are simply supposed to have weaker immune systems.
If you do decide to boil the water when preparing your baby's formula, the FDA recommends that you "bring it to a very bubbly boil. Keep boiling it for a minute or two, then let it cool." Once it has cooled, you will be ready to add it to your baby's formula.
To reduce this risk, the WHO recommends cleaning and sterilizing feeding and preparation equipment and then making a fresh bottle of powdered infant formula for each feed by:
Getting too much fluoride when your child's teeth are still forming can lead to enamel fluorosis, which can cause tooth staining. This staining can appear as faint while markings on a child's baby teeth, and even more importantly, their permanent teeth.
Fortunately, fluorosis is usually very mild when it is caused by fluoridated water and baby formula and the staining is barely noticeable. To reduce your baby's chance of developing even mild fluorosis, it can help to use low-fluoride water (less than 0.7 mg/L) when you prepare your baby's formula, including some types of tap water, and water that has been purified, deionized, demineralized, distilled, or filtered by reverse osmosis.
You don't have to be concerned about fluorosis if you are exclusively or mostly breastfeeding your baby or using a ready-to-feed baby formula.
Sources:
U.S. EPA. Is There Lead in my Drinking Water?
American Academy Of Pediatrics. Heading Home with Your Newborn: From Birth to Reality (Paperback). by Laura A. Jana, Jennifer Shu
Brouard C. Two consecutive large outbreaks of Salmonella enterica serotype Agona infections in infants linked to the consumption of powdered infant formula. Pediatr Infect Dis J - 01-FEB-2007; 26(2): 148-52
Mimouni FB. Bacterial contamination during routine formula preparation. - Am J Infect Control - 01-FEB-2002; 30(1): 44-5
U.S. Food and Drug Administration. Feeding Your Baby with Breast Milk or Formula
CDC. Community Water Fluoridation. Background: Infant Formula and the Risk for Enamel Fluorosis
WHO. Guidelines for the safe preparation, storage and handling of powdered infant formula. http://www.who.int/foodsafety/publications/micro/pif2007/en/ Accessed Dec. 2011.
A. Since few babies like cold baby formula and you do usually have that extra step of warming the formula after you make it, using warm or hot water to start with from the tap does seem like it could save an extra step. You shouldn't do it though.
Instead, follow the directions on your baby's formula package and if using tap water, start with cold tap water. According to the U.S. Environmental Protection Agency, you should "never cook or mix infant formula using hot water from the tap."
What's wrong with hot tap water? Many homes have plumbing with lead or lead solder and hot water can concentrate the lead. Running the water for 15 to 30 seconds and only using cold water can help reduce your baby's exposure to lead from tap water.
Boiling the water doesn't get rid of the lead either. Many home water filters, including pitcher and faucet filters, do remove lead from drinking water though.
Boiling Water???
Another common question about preparing baby formula is whether or not you have to boil the water first. While most brands of baby formula once recommended boiling as a part of their instructions, they now often recommend "asking your baby's doctor or "local health department" instead.The American Academy of Pediatrics doesn't offer any formal advice on the subject either. The latest book on newborns that they published, Heading Home with Your Newborn: From Birth to Reality, does say that "you may want to use boiled or purified (bottled or filtered) water, at least in the first month or two."
The main problem with that statement is that purified, bottled, or filtered water, even the brand Nursery Purified Water, isn't sterile, so isn't necessarily any safer than tap water that hasn't been boiled first. Bottled and filtered water should have fewer impurities and contaminants, including lead, but could still have harmful bacteria, which was the whole reason you were supposed to boil tap water when making baby formula in the first place.
And there is no research which states that doing anything special to the water that you use for your baby's formula "in the first month or two" is helpful or does anything at all. That advice is likely based on the fact that younger babies are simply supposed to have weaker immune systems.
If you do decide to boil the water when preparing your baby's formula, the FDA recommends that you "bring it to a very bubbly boil. Keep boiling it for a minute or two, then let it cool." Once it has cooled, you will be ready to add it to your baby's formula.
WHO Guidelines for Preparing Formula
The World Health Organization issued guidelines on the safe preparation, storage and handling of powdered infant formula after experts recognized that powdered formula was not sterile and was sometimes putting babies at risk for serious bacterial infections.To reduce this risk, the WHO recommends cleaning and sterilizing feeding and preparation equipment and then making a fresh bottle of powdered infant formula for each feed by:
- cleaning and disinfecting all surfaces you will be using and washing your hands properly
- boiling water, even if it is bottled water
- let the water cool (not more than 30 minutes though, so it doesn't get below 70 degrees) and pour it into a cleaned and sterilized bottle
- add the exact amount of powdered formula to the water
- assemble the bottle and mix the powdered formula thoroughly
- quickly cool the bottle by holding it under running tap water or by placing it in a container of cold water or iced water
- dry the bottle with a clean cloth
- check the temperature of the formula so that it doesn't burn your baby's mouth
- feed your baby if the formula is at an appropriate temperature
Baby Formula Safety
After you prepare your baby's formula, you should follow some simple rules to keep your baby safe.- Unless you refrigerated the prepared formula, feed it to your baby within two hours (hold time).
- If you do put the prepared formula in the refrigerator, be sure to use it within 24 hours.
- Once your baby starts feeding from a bottle, be sure he finishes the formula within one to two hours (hang time) and don't put the bottle back in the refrigerator. Unused formula should not be saved for later. Instead, simply prepare less formula next time so that you don't have so much lefted over.
- Don't warm baby formula bottles in the microwave. Instead, use a baby bottle warmer or place the bottles in a container of warm water.
- Follow the baby formula mixing instructions carefully and don't dilute or concentrate the baby formula unless your pediatrician tells you to.
Fluoride and Preparing Baby Formula
Experts often recommend that children should get fluoridated water to help prevent cavities. Surprisingly, infants who are fed powdered or concentrated liquid formula which is mixed with fluoridated water can get too much fluoride.Getting too much fluoride when your child's teeth are still forming can lead to enamel fluorosis, which can cause tooth staining. This staining can appear as faint while markings on a child's baby teeth, and even more importantly, their permanent teeth.
Fortunately, fluorosis is usually very mild when it is caused by fluoridated water and baby formula and the staining is barely noticeable. To reduce your baby's chance of developing even mild fluorosis, it can help to use low-fluoride water (less than 0.7 mg/L) when you prepare your baby's formula, including some types of tap water, and water that has been purified, deionized, demineralized, distilled, or filtered by reverse osmosis.
You don't have to be concerned about fluorosis if you are exclusively or mostly breastfeeding your baby or using a ready-to-feed baby formula.
What You Need To Know
- Talk to your pediatrician to see if you need to boil your water, especially if you are using well water that hasn't been recently tested, or if you aren't convinced that the tap water where you live is safe and healthy for a baby. Your pediatrician can also recommend water -- tap, filtered tap, or bottled water -- that is best for mixing your baby's formula.
- Powdered baby formula is not sterile and there have been cases of Salmonella and Cronobacter sakazakii infections linked to powdered infant formula.
- Boiling water when preparing baby formula is very important in many parts of the world, especially developing countries that do not have safe water supplies.
- Sterile liquid baby formula is recommended for infants in high risk situations, if the babies aren't breastfeeding, especially for premature babies in the NICU.
Sources:
U.S. EPA. Is There Lead in my Drinking Water?
American Academy Of Pediatrics. Heading Home with Your Newborn: From Birth to Reality (Paperback). by Laura A. Jana, Jennifer Shu
Brouard C. Two consecutive large outbreaks of Salmonella enterica serotype Agona infections in infants linked to the consumption of powdered infant formula. Pediatr Infect Dis J - 01-FEB-2007; 26(2): 148-52
Mimouni FB. Bacterial contamination during routine formula preparation. - Am J Infect Control - 01-FEB-2002; 30(1): 44-5
U.S. Food and Drug Administration. Feeding Your Baby with Breast Milk or Formula
CDC. Community Water Fluoridation. Background: Infant Formula and the Risk for Enamel Fluorosis
WHO. Guidelines for the safe preparation, storage and handling of powdered infant formula. http://www.who.int/foodsafety/publications/micro/pif2007/en/ Accessed Dec. 2011.
No comments:
Post a Comment